Objective seizure detection
Real life EEG monitoring provides doctor and patient an objective measure of the seizure burden over time. This new insight can help optimise treatment and management in epilepsy and adds a new tool for the epilepsy community. Our solution is CE marked.
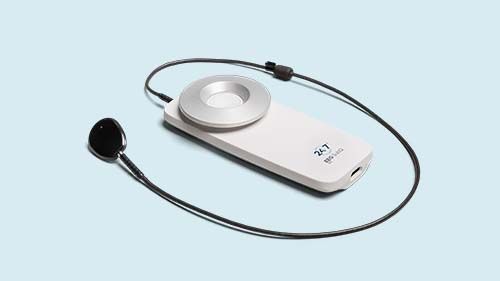
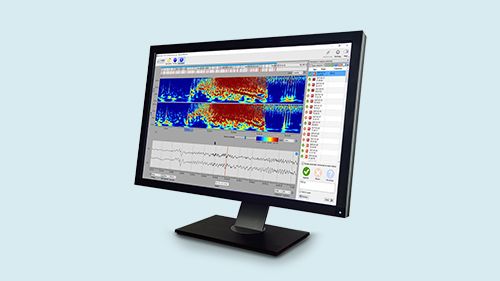
With 24/7 EEG™ SubQ solution we offer 15 months of monitoring, day and night, and on-demand access to patient’s EEG data at your convenience. The solution consists of a subcutaneous implant, a recorder, secure data transfer and EEG analytics.
Our EEG solution
Monitoring epilepsy with the 24/7 EEG SubQ
Murray shares his personal story with epilepsy and what it is like to live with the 24/7 EEG SubQ which records his EEG in daily life and monitors his epilepsy.
Benjamin's epilepsy is monitored 24 hours a day, 7 days a week with the 24/7 EEG SubQ. In this video he explains how he and his neurologist have used the data obtained from the 24/7 EEG SubQ to improve his quality of life.
Highlights
REAL-ASE Study
12/8/2023
We have reached an exciting milestone in the UK: Yesterday, the first patient in the REAL-ASE study had the 24/7 EEG™ SubQ implanted.
Policy Lab workshop
11/17/2023
Our colleagues were in London yesterday to participate at a Policy Lab workshop held at The Policy Institute at King’s College London.
Green Smiley to UNEEG medical
9/19/2023
UNEEG medical has received a Green Smiley from the Work Environment in Denmark.
Breaking new ground in epilepsy
It’s time to change how we understand and manage epilepsy — and create a new standard for patient care. What if you could uncover missing pieces to the puzzle that is epilepsy — together with your patients? What if you could access EEG data more consistently than ever before, and uncover new patterns in your patients’ seizures?
Watch this animation to learn more our ultra long-term EEG monitoring solution.
Looking for a medtech career?

We are always looking for new talent to join us. If you want a career in a rapidly expanding, innovative medtech company, then you should check out our vacant positions or send us an unsolicited application. We looking forward to hearing from you.
Clinical publications
A number of papers on ultra long-term monitoring has been published in a variety of scientific journals. Get access to those clinical publications and posters below.
Contact
UNEEG medical
Borupvang 2
DK-3450 Allerød
Denmark
Phone: +45 4063 8000
Mail: uneeg@uneeg.com
VAT-NO: DK29140774
CVR-NO: 29140774
Contact us